Renewed Hope: China's Advancing ADC Therapies and the 2025 Approvals Illuminating the Path for Cancer Warriors
In a world where cancer remains a formidable enemy, every scientific advance becomes a beacon of hope for millions of patients fighting day by day. As an editor at DengYueMed, a company that, with its commitment to excellence, innovation, sustainability, and social responsibility—built on the foundations of quality, compliance, and integrity—actively participates in the global pharmaceutical market landscape, not only helping innovative Chinese medicines expand overseas but also contributing to the health and well-being of people worldwide, I have closely followed how innovations in oncology treatments are transforming what once seemed inevitable into real possibilities for remission and improved quality of life. This is not just about cold data or statistics; it is about human stories, about the courage of those facing exhausting chemotherapies, invasive surgeries, and constant uncertainty. Today, I want to share with you how the surge in China's antibody-drug conjugate (ADC) pipeline, with key approvals in 2025, is igniting new light for those battling solid tumors such as breast, lung, or ovarian cancer.
Let us imagine for a moment the journey of a patient with HER2-positive breast cancer. Traditional options, though valuable, often come with side effects that erode physical and emotional strength. But in recent years, China has emerged as a leader in ADC development—therapies that combine precise antibodies with potent drugs to directly target cancer cells while minimizing damage to healthy ones. According to recent reports, hundreds of these compounds are in advanced clinical stages in the country, focusing on antigens such as HER2, EGFR, Trop-2, c-Met, and CDH6. Companies like Kelun-Biotech and RemeGen are at the forefront: for example, drugs like Jiataile, Datroway, and Aidixi have shown promising results in trials, offering objective response rates exceeding 50% in some cases of resistant cancers.
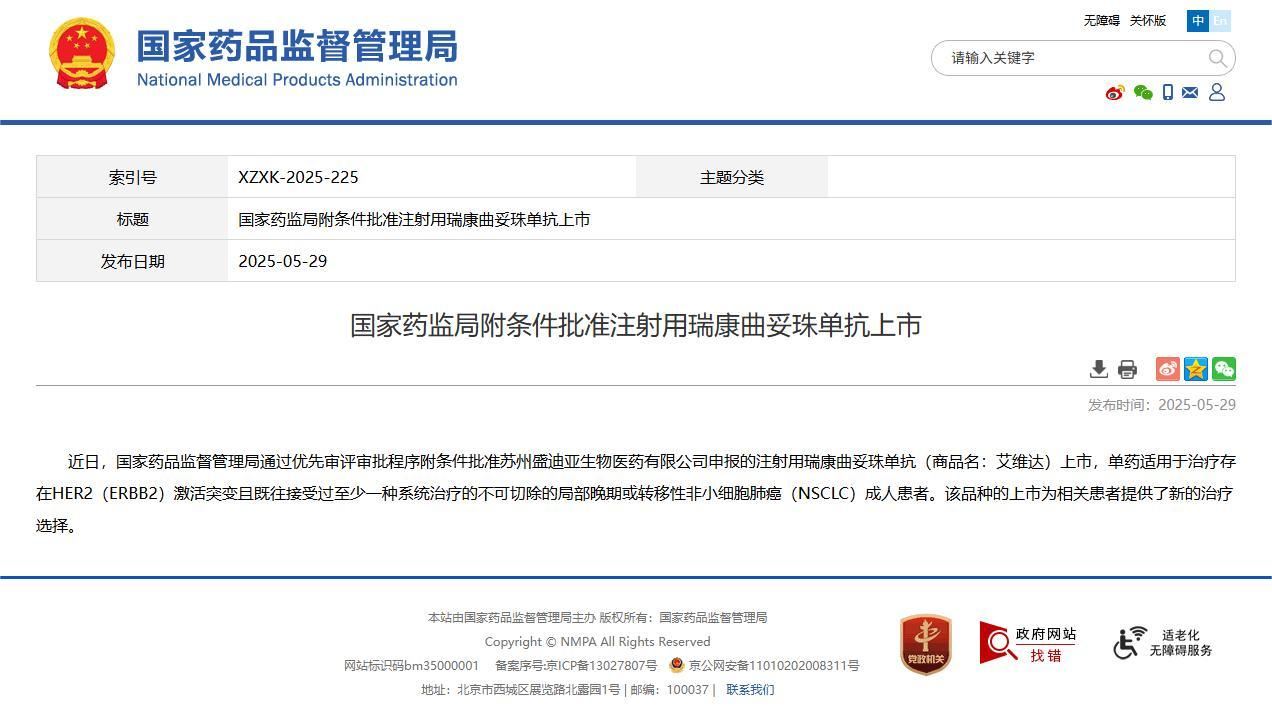
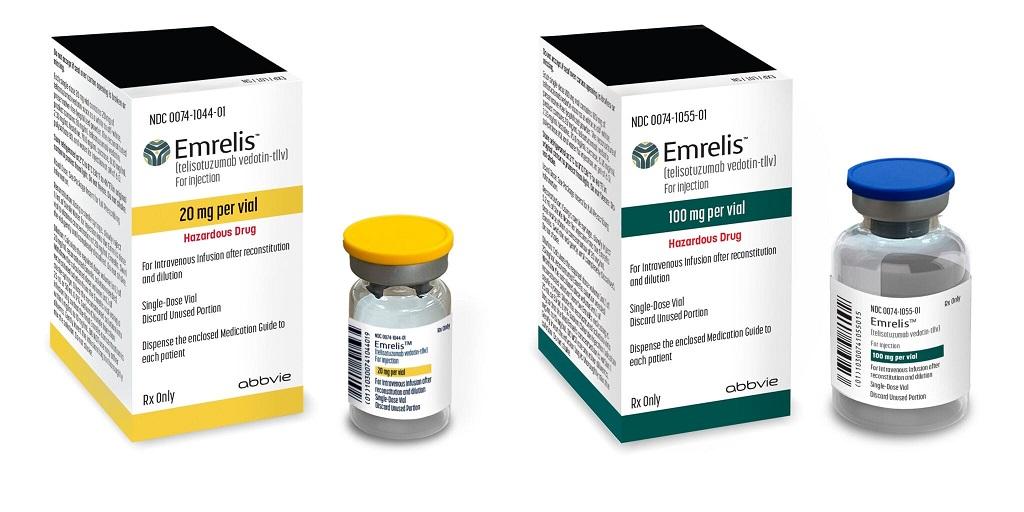
What moves me most, from my perspective at DengYueMed, is how these advances are not mere theoretical promises but concrete realities, with 2025 approvals that are changing the global landscape. Take trastuzumab rezetecan, targeted at HER2-positive cancers, approved in May by China's NMPA, or Enhertu (trastuzumab deruxtecan), which received approval in December for first-line treatments in metastatic HER2-positive breast cancer. In non-small cell lung cancer (NSCLC) with EGFR mutations, Datroway (datopotamab deruxtecan) was approved in June for advanced cases after prior therapies, promising to extend remissions in patients who have exhausted other options. And let us not forget Emrelis for NSCLC with high c-Met expression, approved in May, or SystImmune's iza-bren, a bispecific ADC that in phase 1 has demonstrated antitumor activity in pretreated solid tumors, with manageable safety profiles.
These developments resonate deeply with me because, in my editorial role, I have read patient testimonials describing cancer not only as a physical illness but as an emotional battle where hope becomes the most powerful weapon. I recall the story of a woman with platinum-resistant ovarian cancer: therapies like raludotug deruxtecan, focused on CDH6, have achieved response rates of 50.5% in phase 2/3 studies, with complete remissions in some cases and disease control rates of 77.6%. For recurrent endometrial cancer, rinatabart sesutecan has reached disease control rates close to 100% at low doses, with objective responses of 50%. Is this not a reminder that, even in the darkest moments, innovation can offer respite?
However, from my point of view, these advances also invite us to reflect on human resilience. They are not miraculous cures, but they represent a step toward more personalized treatments with less toxicity and greater efficacy. In China, this wave of research not only addresses local needs but also contributes to the global effort against cancer, reminding us that scientific collaboration transcends borders. For patients, this means not just surviving, but living with dignity: being able to watch their children grow, enjoy a sunset without the constant weight of fear.
Dear readers, if you are in this fight or supporting someone who is, know that you are not alone. The progress in Chinese ADCs in 2025 is not just news; it is testimony that hope is renewed with every discovery. Keep the faith, consult your doctors, and embrace every small victory. Tomorrow looks brighter, and together, we will move forward.
By the editorial team at DengYueMed
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jocuri
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Alte
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
